GM6 and ALS
ALS Remains a Devastating and Incurable Disease
Amyotrophic Lateral Sclerosis (ALS) is a progressive neurodegenerative disease that affects nerve cells in the brain and spinal cord. It usually strikes people between the ages of 40 and 70, and approximately 21,000 people in the U.S. have the disease at any given time with approximately 5,000 people diagnosed each year. Average life expectancy is 2-5 years from initial diagnosis. ALS is designated a rare disease for which GM604 has received orphan drug designation in both the U.S. and in Europe.
ALS therapies aimed at single molecular targets have not been successful. There are currently only two approved therapeutics: riluzole (Rilutek) and edaravone (Radicava). These current therapies have limitations and may only prolong survival for a few months. No cure currently exists.
With its novel multi-target mechanisms of action, GM6 could modify neurodegenerative pathways and reverse existing pathology to treat ALS.
To evaluate the disease modification potential of GM6 in ALS, GM6 was studied in a Phase 2A clinical trial. This trial recruited fast-progressing, definite ALS patients within a 2-year onset. There were no clinically significant drug-related serious adverse event. GM6 showed favorable shifts in the following plasma biomarkers with statistical significance: SOD1, Tau, and TDP-43. There were positive signals of GM6 slowing FVC decline and slowing functional decline as measured by the ALSFRS-R rating scale. See detailed results below.
ALS Phase 2A trial was completed and is Registered under Clinicaltrials.gov - NCT01854294.
GM6 is phase 3 ready for ALS trial.
GM6 INCREASES NEUROGENESIS AND LOWERS SOD1 AND INFLAMMATION TO AMELIORATE ALS DISEASE IN ALS SOD1 ANIMAL MODEL
In an ALS SOD1 mice model, in a dose dependent fashion, GM6 improved behavior, survival rate, strength, and clinical score with statistical significance.
In addition, GM6 significantly increased motor neuron survival, reduced hSOD1 protein level, and reduced inflammation biomarkers.
At 1 mg/kg and 5 mg/kg, reduction of cytokines were: TNFα (-50% and -60%), IL-1β (-65% and -80%), and TGF-β (-50% and -65%). Increased NGF (+60% and +160%)
GM6 may modulate ALS disease through regulating inflammation response and increasing NGF.
ASENT 2023 Annual MeetinG
Dr. Mark S. Kindy, Ph.D., FAHA
GM6 Impact on TDP-43 levels in human TAR4 tg mice may be therapeutic for ALS, and frontotemporal lobar degeneration (FTLD)
Click on video to view
GM604 - ALS Poster
GM604 Phase 2A Paper Published
Safety and Efficacy Summary of GM604
Genervon’s research team summarized the mechanisms of actions of GM6 for ALS.
The ALS MOA summary can be accessed at this link. For the full report, please contact info@genervon.com.
GM6 and
Parkinson’s Disease
Parkinson’s disease affects about 1 million people in the United States and 10 million worldwide
Parkinson’s usually affects individuals aged 60 and older as age is the largest risk factor. Parkinson’s disease itself is not fatal, but disease complications can be serious.
The cause of Parkinson’s disease (PD) is the loss of dopamine. GM6 does not act as an exogenous source of dopamine. Rather, GM6 protects the dopaminergic neurons in the Substantia Nigra Pars Compacta (SNpc) from dying which consequently increases dopamine. GM6 strongly reduced neuroinflammation, reduced α-synuclein deposits by down-regulating SNCA, and increased BDNF significantly as demonstrated in the Phase 2A clinical trial.
PD Phase 2A trial was completed and is Registered under clinicaltrials.gov - NCT01850381.
The PD Phase 2A clinical trial demonstrated that GM6 is safe and may slow the rate of clinical worsening of PD.
After 6 doses of GM6 at the end of 2 weeks, the level of neuroprotective biomarker BDNF in the GM6-treated group was higher than in the placebo group with statistical significance.
Changes in four of the eight secondary endpoints (UPDRS ADL, Schwab&England, Hoehn&Yahr, and MOCA) when compared with placebo were statistically significant.
No serious adverse events were recorded. No clinically significant lab test results difference longitudinally.
GM6 is Phase 2B ready for PD trial.
GM6 IN MPTP AND 6-OHDA PD ANIMAL MODELS IMPROVED FUNCTIONS AND MOTOR ACTIVITIES AND INCREASED DOPAMINE AND NEURON PROTECTION
Intravenous injections of varying doses of GM6 of 1, 5, 10, or 20 mg/kg in saline showed dose response significant improvements in behavioral testing including spontaneous activity and time-to-rotarod failure with statistical significance.
Quantification of brain monoamines with high pressure liquid chromotography (HPLC) showed GM6 significantly increased Monoamine (dopamine [DA] and metabolite dihydroxyphenyacetic acid [DOPAC] and homocanillic acid [HVA] levels in both models.
The figures below show that GM6 increases dopaminergic neurons in the Substantia Nigra Pars Compacta (SNpc) in 6-PHDA mouse Model for PD by Immunohistochemical staining.
GM6 protects the dopaminergic neurons in SNpc from the detrimental effects of PD induction.
Genervon’s research team summarized the mechanisms of actions of GM6 for Parkinson’s Disease.
The PD MOA summary can be accessed at this link. For the full report, please contact info@genervon.com.
GM6 and
Alzheimer’s Disease
Alzheimer’s Disease is a worldwide health crisis.
Approximately 44 million people worldwide have Alzheimer’s disease (AD) or a related dementia. Every 65 seconds, someone in the United States develops Alzheimer’s. Alzheimer’s usually affects individuals aged 65 and older but can strike earlier as young-onset Alzheimer’s. The average life expectancy is 8 to 10 years from initial diagnosis.
All Phase 3 clinical trials of drugs using the reduction of Aβ as the therapeutic target have failed. Alzheimer's disease is a multi-factorial central nervous system disorder involving complex pathologies, and treatment requires simultaneous regulation of multiple pathogenesis.
The current AD drug development pipeline targets only a single mechanism from the list of multiple pathogenesis in AD disease such as reducing amyloid, reducing tau, reducing inflammation, reducing synaptic plasticity/increasing neuroprotection, modulating vasculature complication, and modulating metabolism/bioenergetics, etc.
Combination therapy using multiple drugs has been proposed for AD treatment. However, Genervon proposes the use of a drug with multiple effects in one molecule. GM6 is such a pleiotropic and endogenous regulator that simultaneously regulates multiple pathological pathways and genes to treat Alzheimer’s disease.
In animal models, GM6 has shown its potential to treat AD by simultaneously reducing Aβ amyloid, tau, hyperphosphorylated tau, TDP-43, fibrinogen, and inflammation and increasing nerve growth factor for neuroprotection. See posters and presentations below.
Genervon is ready for Phase 2 clinical trials for AD.
GM6 ATTENUATES AD PATHOLOGY IN APP MICE IN MULTIPLE PATHWAYS BY REDUCING A𝝱 PEPTIDE AND INFLAMMATION AND MODULATING KEY BIOMARKERS
Genervon investigated the effect of GM6 in 3-month old APP/ΔPS1-Tg double–transgenic mice model before the development of amyloid plaques at ~3 to 4 months.
GM6 treatment demonstrated a dose-dependent, statistically significant (P < 0.05), and favorable outcome in APP mice after 4 months of treatment with 1 or 5 mg/kg of GM6 compared to placebo, with 5mg/kg results as follows with statistical significance:
Reduced accumulation of Aβ peptide ▼80%
Reduced amyloid load ▼71% Aβ₁₋₄₂, ▼66% Aβ₁₋₄₀
Reduced Inflammation ▼84% TNFα, ▼93% IL-1β, ▼93% TGF-β, ▼microglia 70%, ▼astrocytes 71%
Decreased cathepsin B expression ▼62%
Improved spatial orientation by faster travel: ▼escape latency time and distance traveled 56%
Increased NGF levels in brain ▲ 600%, and
Tempered the memory impairment ▼50%
GM6 modulates various pathways early in the disease process including up-regulating APP catabolism to reduce Aβ deposit and effectively alter the disease process in AD.
GM6 ATTENUATES AD PATHOLOGY IN H-TAU MOUSE MODEL BY REDUCING INFLAMMATION, LOWERING TAU HYPERPHOSPHORYLATION, AND ATTENUATING BEHAVIORAL CHANGES
In healthy neurons, tau normally binds to and stabilizes microtubules. Abnormal chemical changes cause tau to hyperphosphorylate and form tangles which block the neurons’ transport system causing tauopathy and clinical manifestations of AD. Tau is a downstream target of GM6 as manifested in the lowering of the hyperphosphorylation of tau.
In vitro study with SK-N-SH cells treated with AGE-BSA or aggregated Aβ₁₋₄₂ showed a dose-dependent increase in tau hyperphosphorylation as determined by Western blot analysis of pT231. GM6 treatment reduced pT231 by >50%.
In a mouse tau model (h-Tau) which showed hyperphosphorylation of h-Tau and behavioral deficits, GM6 demonstrated a reduction of pT231 hyperphosphorylated tau (>75%) and attenuated the behavioral changes with statistical significance (>50%) (n=10 animals/group).
Cytokines were extracted from h-Tau mouse brains and measured by ELISA. Quantitative analysis of cytokine levels by ELISA for the vehicle control group compared to the GM6-treated group showed a statistically significant decrease in TNF-α at 60 vs 10pg/mg protein (-83%, p<0.05), in IL-1β at 180 vs 20 pg/mg protein (-89%, p<0.05), and in IL-6 at 120 vs 20 pg/mg protein (-83%, p<0.05).
DNA microarray study indicated that GM6 represses tau (MAPT↓) and down-regulates mitochondrial genes.
GM6 Can Block Tau Hyperphosphorylation Induced by AGEs and A𝝱
ASENT 2022 Annual MeetinG
Dr. Mark S. Kindy, Ph.D., FAHA
GM6 attenuates activated cofilin and β-arrestin2 impact on pathological tau and decreasing tau in Alzheimer’s disease (AD) and frontotemporal lobar degeneration (FTLD)
Click on video to view
ASENT 2021 Annual MeetinG
Dr. Mark S. Kindy, Ph.D., FAHA
GM6 Attenuates Inflammation in Alzheimer’s Disease Pathology Via Modulation of Fibrinogen by Reducing Aβ and Tau
Click on video to view
Genervon’s research team summarized the mechanisms of actions of GM6 for Alzheimer’s Disease.
The Alzheimer’s disease MOA summary can be accessed at this link. For the full report, please contact info@genervon.com.
GM6 and Stroke
Stroke is the 4th leading cause of death, costing the U.S. an estimated $73 Billion each year.
The only approved drug available is tPA for blood clots dissolving within 6 hours. Single mechanism drugs have failed. The other treatment for acute ischemic stroke is by a mechanical thrombectomy device to remove the blood clots. Two issues may limit the widespread clinical use of mechanical thrombectomy. First, only an estimated 10 percent of patients with acute ischemic stroke have a proximal large artery occlusion in the anterior circulation and present early enough to qualify for mechanical thrombectomy within 6 hours. Secondly, only a few stroke centers have sufficient resources and expertise to deliver this therapy. There is a need for treating stroke patients after 24 hours of onset. Growth factor is one of the strategies that can help stroke patients recover. GM6 is an active fragment of motoneuronotrophic factor (MNTF), a growth factor that is endogenously expressed at the embryonic stage which provides neuroprotective and neuroregenerative therapeutic effects. GM6 is proposed as a potential therapy for stroke recovery.
The neuroprotective functions of GM6 were shown in stroke models. In multiple Occlusion of Middle Cerebral Artery (MCAO) mouse models, GM6 decreased infarct and neurological deficit in a dose-dependent manner with statistical significance. See published paper PMID18789909.
GM6 is Phase 2B ready for Stroke.
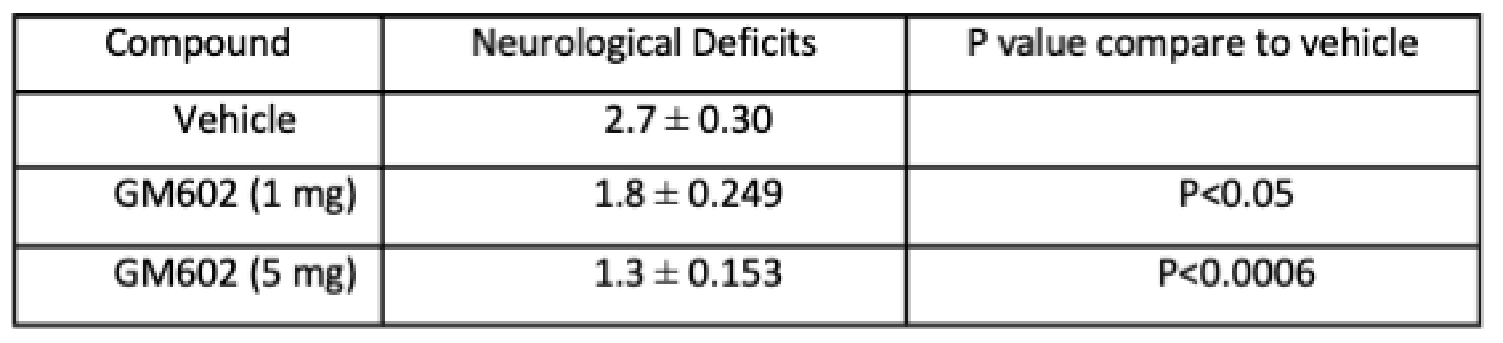
GM6 DECREASES INFARCT AND NEUROLOGICAL DEFICIT IN MCAO STROKE MODEL*
1 hour occlusion of middle cerebral artery (MCAO) of mice, drug given IV at beginning of 24-hour reperfusion
Treatment with GM6 reduced infarct size in a dose-dependent manner with statistical significance.
Neurological scores were as follows:
0, normal motor function;
1, flexion of torso and contralateral forelimb when animal was lifted by the tail;
2, circling to the contralateral side when held by tail on flat surface, but normal posture at rest;
3, leaning to the contralateral side at rest;
4, no spontaneous motor activity.
Treatment with GM6 improved motor function as seen in the statistically significant reduction in neurological deficit scores in a dose-dependent manner.
GM6 and
Multiple Sclerosis
Multiple Sclerosis (MS) is a potentially disabling disease of the brain and spinal cord.
In MS, the immune system attacks the protective sheath (myelin) that covers nerve fibers and causes problems with communication between the brain and the body. Symptoms include: Visual: visions loss, Sensory: pain and needles, excessive urination, Speech: slurred speech, Muscular: cramping, rigidity, difficulty walking, spasms, impaired coordination, tremor, whole body fatigue.
MS is a rare disease. There are fewer than 200,000 cases in the U.S. per year.
In animal models, GM6 demonstrated modulation of MS and improvement in motor functions. GM6 reduced clinical signs and reduced lesions in the brain and spinal cord in Experimental Autoimmune Encephalomyelitis (EAE)-induced MS mouse model with statistical significance in a dose-dependent manner.
Genervon is ready for Phase 2 clinical trials for MS.
GM6 Improved Clinical Score in Multiple Sclerosis Mice Models
Mean clinical scores in mice following MS induction and GM6 treatment:
GM6 is effective in limiting the extent of MS, a neurodegenerative/ autoimmune disease.
GM6 Effect in EAE-induced MS model:
Reduction of Lesions
The number of lesions in the brain and spinal cord were determined by counting and are shown in the table above.
As the dose of GM6 increased, the number of lesions in the brain and spinal cord decreased. This suggests that GM6 protects the brain and spinal cord from the detrimental effects of MS induction.
GM6 is effective in limiting the extent of MS in mouse via intravenous administration and may be beneficial for treating this disease.
GM6 and
Spinal Cord Injury
Spinal cord injury patients require multidisciplinary care management such as the reduction of pain, prevention of further damage to the spinal cord, and improvement of pathophysiological mechanisms such as glial scar formation, demyelination, and astrogliosis. Emerging drugs for spinal cord injury recovery in clinical trials include the use of human growth factor, recombinant human acidic fibroblast growth factor (rhFGF1), and other stimulants for neurogenesis, antioxidants, chemokine inhibitors, etc.
GM6, being an active fragment of Motoneuronotrophic factor (MNTF), has the potential to protect and improve neuro-regeneration. In the spinal cord injury (SCI) animal model, GM6 demonstrated a dose-dependent significant reduction in lesion volume and improvement in motor functions. These data suggest that GM6 is neuroprotective in the spinal cord following IV injection in the mouse model of SCI.
Genervon is ready for Phase 2 clinical trials for SCI.
GM6 Reduces Lesion Volume and Improves Motor Functions in Spinal Cord Injury (SCI) Model
Images below show that the damaged area of the spinal cord following SCI in the GM6-treated animal (B) is significantly smaller compared to control (A).
A. Vehicle treated mouse.
B. Mouse treated with 5 mg/kgGM6.
Rota-Rod Treadmill test on animals subject to SCI:
Open-Field behavioral measurements in mice subject to SCI: